Introduction: LR-MDS are characterized by ineffective erythropoiesis that leads to anemia and red blood cell (RBC) transfusion dependence. Luspatercept is a first-in-class erythroid maturation agent that binds to select TGF-β superfamily ligands and enhances late-stage erythropoiesis. MEDALIST is a phase 3, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of luspatercept in pts with LR-MDS (IPSS-R-defined Very low-, Low-, and Intermediate-risk) with ring sideroblasts who required RBC transfusions and were ineligible for, intolerant of, or refractory to erythropoiesis-stimulating agents. Clinical benefit (CB; defined as RBC transfusion independence [RBC-TI] ≥ 8 weeks and/or modified hematologic improvement-erythroid [mHI-E] per IWG 2006 criteria) in the primary MEDALIST treatment phase (Weeks 1-24) was achieved by 58.2% of pts in the luspatercept arm and 21.1% in the placebo arm (P < 0.0001). The objective of the study was to investigate the effect of luspatercept treatment on erythropoiesis biomarkers and their relationship to CB in the primary MEDALIST treatment phase (Weeks 1-24).
Methods: In the MEDALIST trial, 229 pts were randomized to receive either luspatercept (N = 153) or placebo (N = 76). Reticulocyte count was determined in blood samples collected at baseline and during the primary treatment phase. Serum biomarkers (soluble transferrin receptor 1 [sTfR1], erythroferrone [ERFE], and erythropoietin [EPO]) were measured by ELISA. Bone marrow (BM) erythroid precursors (EP) were determined by cytomorphology from BM aspirates. Biomarker levels were compared between baseline and Week 25 within treatment arms and between pts with CB and without CB in the luspatercept arm using a paired 2-tailed t-test and unpaired t-test (parametric method).
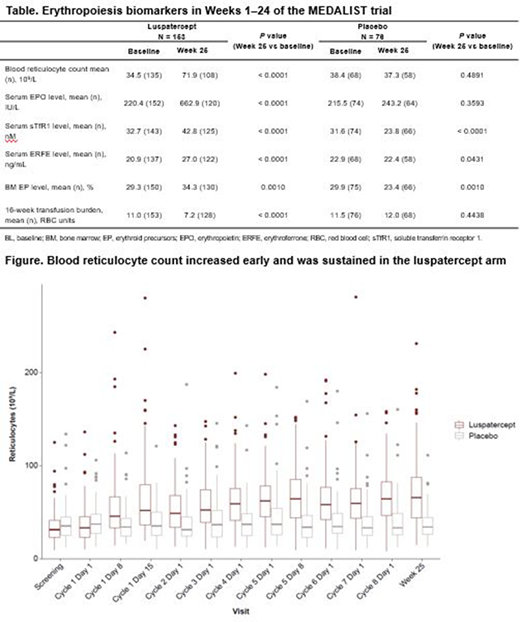
Results: In the luspatercept arm, mean reticulocyte count increased from baseline, starting at 8 days after first dose (55.1 vs 34.5 × 109/L at baseline, P < 0.0001), and remained elevated throughout the evaluation period (Figure). Mean EPO levels increased significantly within 6 weeks after first dose (440.1 vs 220.4 IU/L at baseline, P < 0.0001) and remained elevated up to Week 25. Similarly, levels of sTfR1 (P < 0.0001), ERFE (P < 0.0001), and EP (P = 0.0010) were elevated at Week 25 relative to baseline (Table). The mean transfusion burden (within 16 weeks) was significantly reduced at Week 25 compared with baseline (7.2 vs 11.0 units, P < 0.0001).
In contrast, in the placebo arm, reticulocyte count, EPO levels, and 16-week transfusion burden remained largely unchanged, while levels of sTfR1 (P < 0.0001), ERFE (P = 0.0431), and EP (P = 0.0010) were significantly lower at Week 25 relative to baseline.
In the luspatercept arm, mean baseline EP were higher in 87 pts with CB (31.3%) compared with 63 pts without CB (26.5%; P = 0.0298). No statistically significant differences in baseline EPO, ERFE, sTfR1, reticulocyte count, and 16-week transfusion burden were observed in either group.
At Week 25, pts with luspatercept and CB had a significantly greater increase of reticulocyte count (2.7 vs 1.8 mean fold increase from baseline, P = 0.0017), but not EPO levels (2.9 vs 4.3 mean fold increase from baseline, P = 0.1370) compared with pts without CB. Changes in erythropoiesis-related biomarkers (EP, ERFE, and sTfR1) did not differ significantly between pts with and without CB.
To investigate whether luspatercept affects erythroid maturation, the ratio of reticulocyte/sTfR1 was calculated. This ratio was reasoned to be an approximation of the ratio of late-stage erythropoiesis (reticulocytes) within total erythropoiesis (sTfR1). Luspatercept increased the mean ratio of reticulocyte/sTfR1 in pts with CB (2.2 in Week 25 vs 1.5 at baseline, P < 0.0001) and no CB (1.9 in week 25 vs 1.3 at baseline, P = 0.0071).
Conclusions: Luspatercept-treated pts in the MEDALIST trial had an increase of erythropoiesis-associated biomarkers. Luspatercept-mediated CB (RBC-TI ≥ 8 weeks and/or mHI-E) was associated with increased blood reticulocyte counts and was higher in pts with expanded BM erythropoiesis (as measured by EP) at baseline. Together with the observation that the ratio of reticulocytes/sTfR1 increased during luspatercept treatment, this suggests that the luspatercept mechanism of efficacy in pts with LR-MDS is associated with an increase of erythroid maturation and reticulocytes.
Platzbecker:Novartis: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Amgen: Honoraria, Research Funding. Zhu:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Ha:Bristol Myers Squibb: Current Employment. Risueño:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: Named in BMS (before Celgene) patent filings related to predictive patient response biomarkers in hematological malignancies. Chan:Bristol Myers Squibb: Current Employment. Zhang:BMS: Current Employment. Dunshee:Bristol Myers Squibb: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Genentech Inc.: Current Employment, Current equity holder in publicly-traded company. Acar:Bristol Myers Squibb: Ended employment in the past 24 months. Shetty:BMS: Current Employment, Current equity holder in publicly-traded company. Ito:BMS: Current Employment, Current equity holder in publicly-traded company. MacBeth:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Santini:Menarini: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Acceleron: Consultancy; Novartis: Consultancy, Honoraria; Johnson & Johnson: Honoraria; BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Garbowski:Imara: Consultancy; Vifor Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees. Fenaux:BMS: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Schwickart:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal